Information for Laboratories about Coronavirus (COVID-19)
Healthcare providers* considering testing people with possible COVID-19 should work with their local and state health departments to coordinate testing through public health laboratories, or work with commercial or clinical laboratories using viral tests granted an Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration.
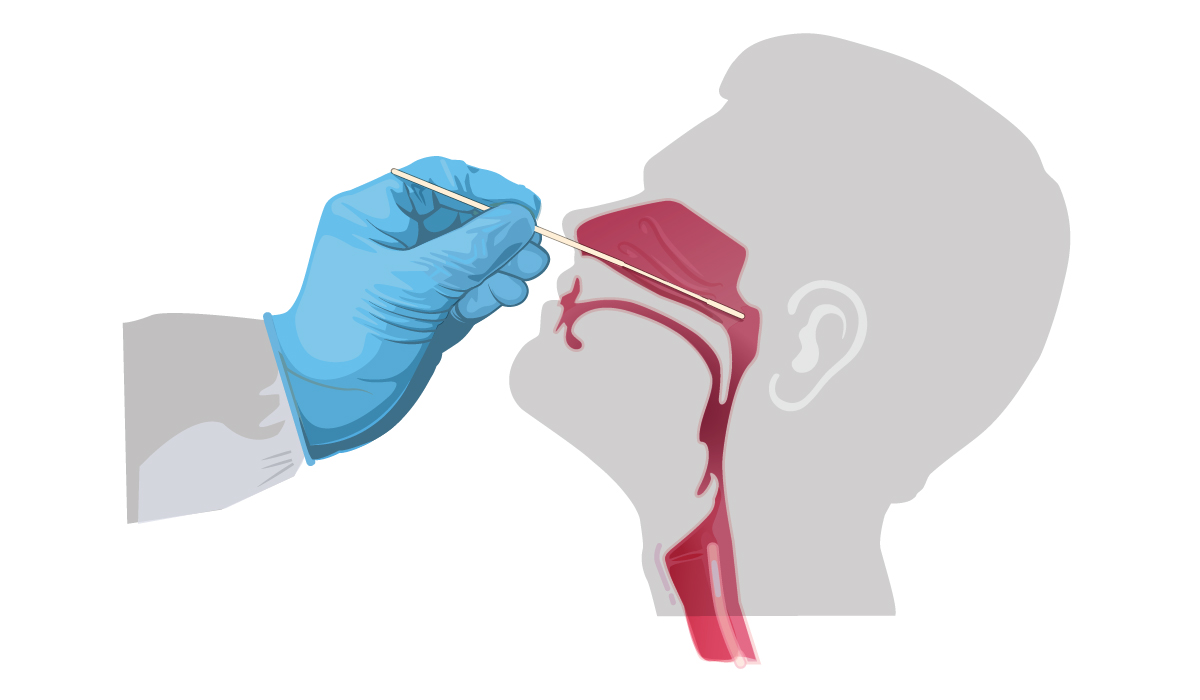
This resource outlines the CDC’s interim guidelines for collecting, handling, and testing clinical specimens for Covid-19. Proper collection of specimens is the most important step in the laboratory diagnosis of infectious disease, especially because a specimen that is not collected correctly may lead to false negative test results. For more information, including illustrations and step-by-step guidance, check out this resource, which is continuously updated to reflect any changes.